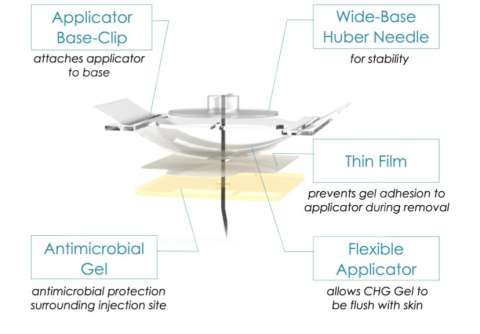
Engineering Innovation in Health (EIH) promotes interdisciplinary collaborations between engineers and a wide range of clinicians with the goal of developing technical solutions to pressing challenges in health care.
We invite any health care professional to submit an unmet health challenge. Previous EIH projects have involved more than 100 clinicians, with diverse training and specialties, including medical doctors, surgeons, nurses, physical therapists, dentists, pharmacists, and pathologists.
Involvement
Submitting your unmet need
The EIH process starts with submitting an unmet health challenge and ultimately ends with a working prototype solution, which can take the form of a device, process, or application.
It takes only a few minutes to submit your initial unmet challenge via the Clinical Project Application. The applications asks you to describe the unmet health challenge, how the challenge is currently addressed, and your vision for how the project might move forward.
Selection process
In late summer (August/September), EIH invites a select group of submissions to give a reverse pitch, describing their unmet needs to our selection committee. The projects that the committee selects move forward in the first phase (October-December) that focuses on deep and holistic understanding of the unmet health challenge.
We often receive more than 50 submissions each year. Typically 10 to 15 projects are invited to participate in the autumn quarter class, and 6 to 8 projects move forward during winter and spring quarters for full development.
Time commitment
The submitting clinician (or team of clinicians) will form a team with engineering students and faculty. Autumn quarter requires roughly 1 to 3 hours per week of clinician time plus attendance at the Fall Showcase in December. If your project is selected for full development during winter and spring quarters (January–June) the time commitment will increase marginally.
There are no direct costs to the participating clinicians; however, we always welcome direct support to the program from individuals, departments, and industry.
Benefits
Prototype solution and intellectual property
If your project is selected for full development, you will receive the solution to your health challenge in the form of a working prototype (device, process, app) in June. You and your team will submit the invention to CoMotion for further consideration for a U.S. patent application. A large percentage of EIH projects have pursued patent applications.
Preliminary data and comprehensive report
You will receive a comprehensive report that covers the background of the unmet need, the existing approaches and technologies that address the challenge, the regulatory pathway, preliminary market opportunity, and background intellectual property, as well as several solution designs and their preliminary data.
Future opportunities
The solution and report provides an abundance of opportunities to move the project forward:
- Submit a patent for the innovation.
- Use the report information and preliminary data gathered for subsequent publications and grant applications.
- Evaluate the innovative solution in the clinic (with appropriate IRB and FDA considerations).
- Begin the process of spinning out a start-up company.